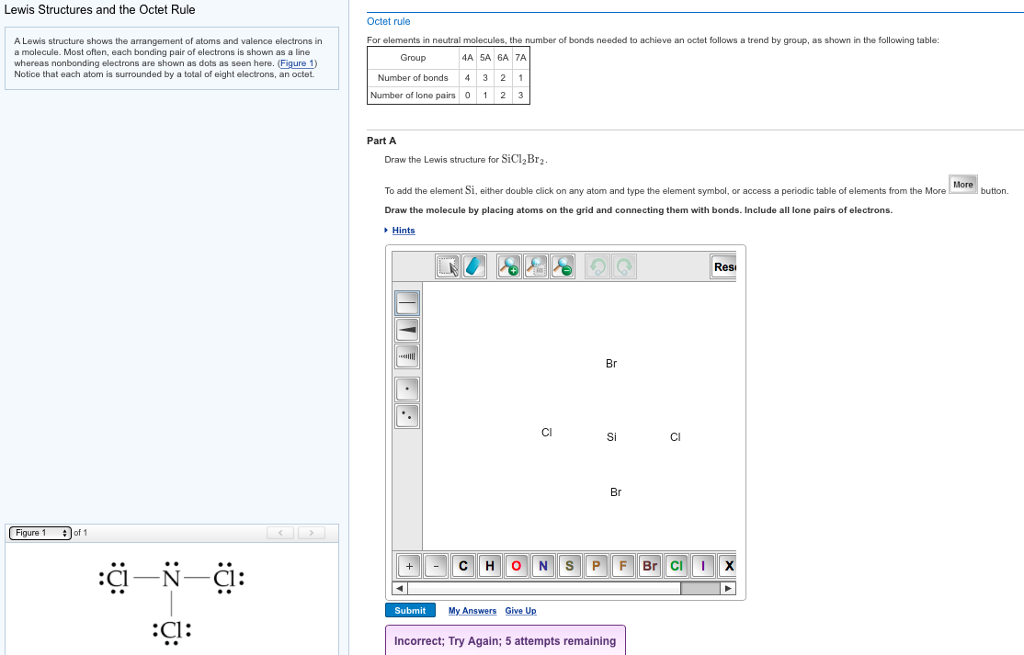
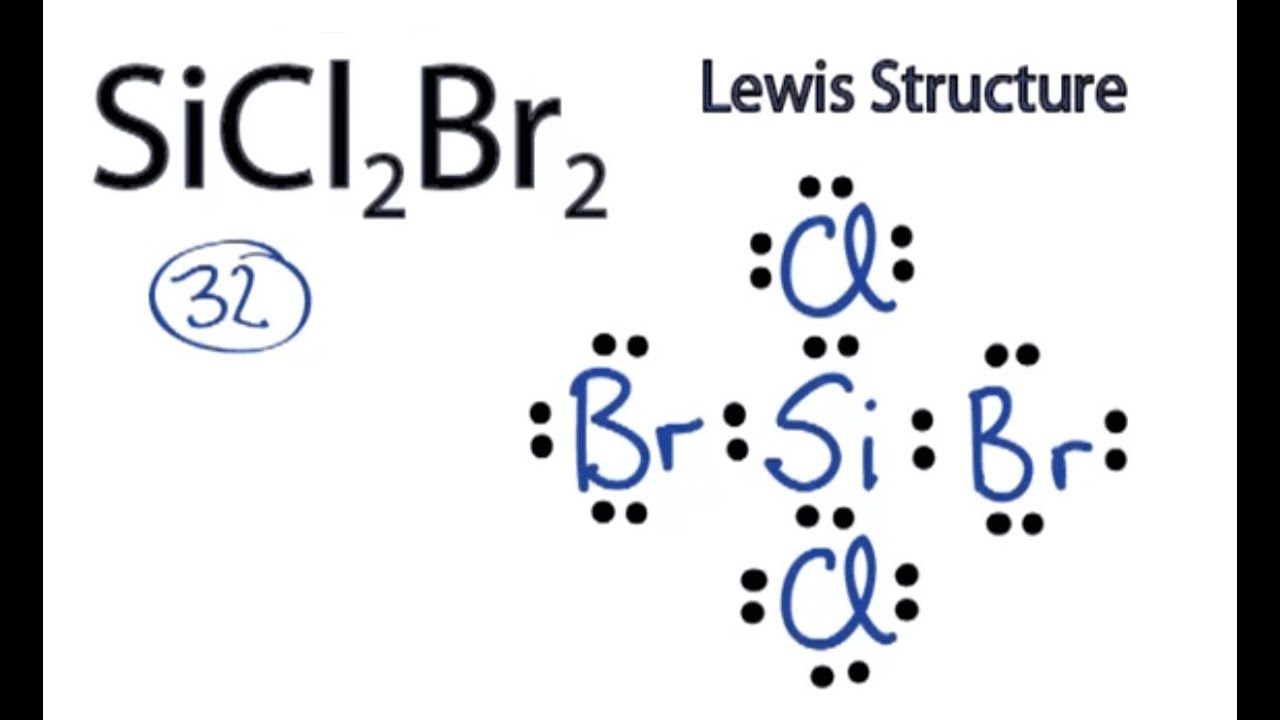Draw The Lewis Structure For Sicl2Br2
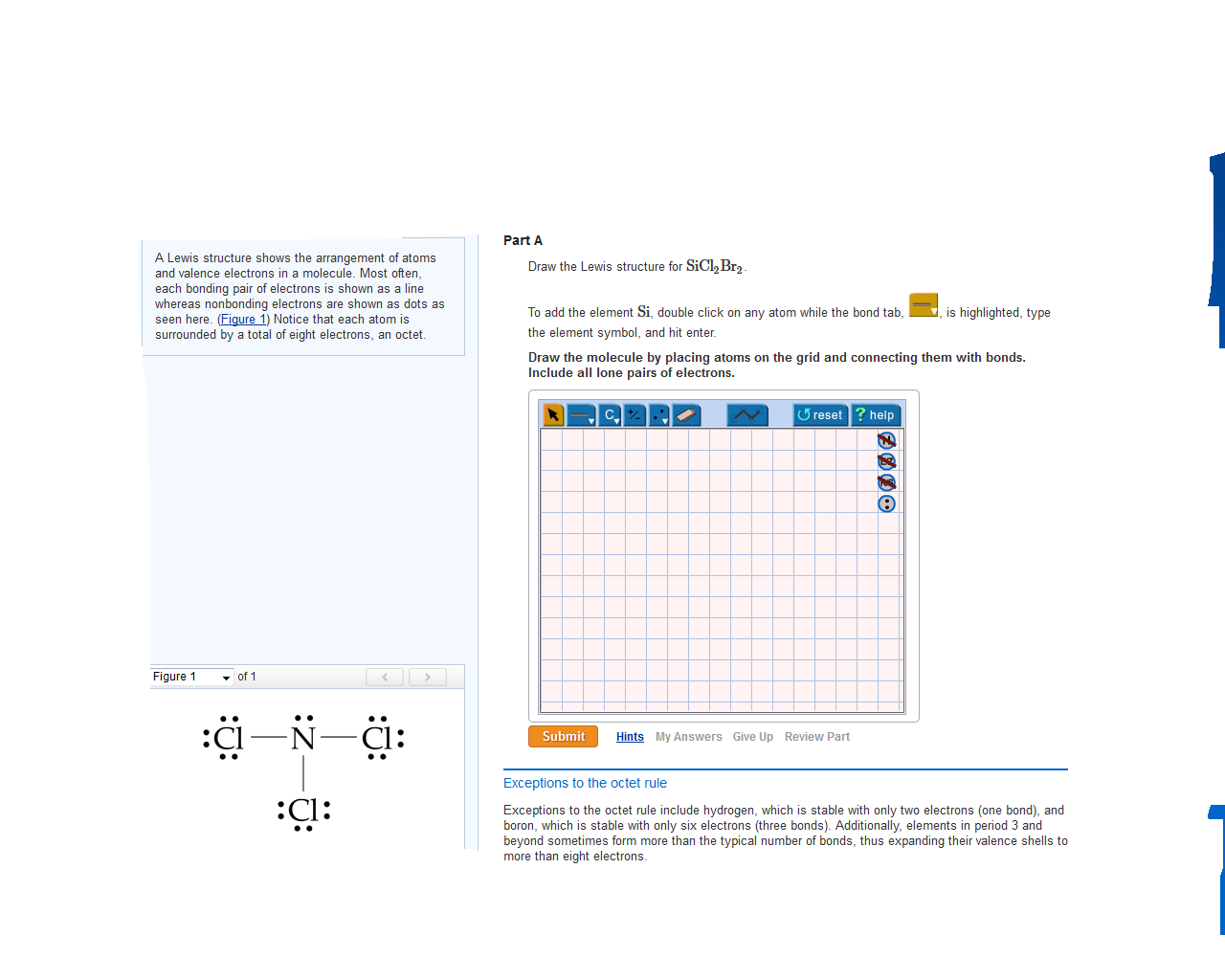
Draw The Lewis Structure For Sicl2Br2 - Sicl 2 br 2 is a tetrahedral compound with sp 3 hybridization. Valence electronic structures can be visualized by drawing lewis symbols (for atoms and monatomic ions) and lewis structures (for molecules and polyatomic ions). Include all lone pairs of electrons. Draw the molecule by placing atoms on the grid and connecting them with bonds. Lewis structures are very similar to electron dot diagrams except for the fact that shared electrons between atoms are shown as lines. Draw the molecule by placing atoms on the grid and connecting them with bonds. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Then, connect si with cl and br atoms using single bonds, which uses up 8 out of 32 total valence electrons. Here, the given molecule is sicl2br2. In order to find the total valence electrons in a sicl2br2 molecule, first of all you should know the valence electrons present in silicon atom, chlorine atom as well as bromine atom. To add the element si, either double click on any atom and type the element symbol, or access a periodic table of elements from the more more button button. This is done by adding the valence shell electrons of all the constituent atoms. Web sicl2br2 is a chemical formula for dibromo dichloro silane. Find more chemistry widgets in wolfram|alpha. In order to find the total valence electrons in a sicl2br2 molecule, first of all you should know the valence electrons present in silicon atom, chlorine atom as well as bromine atom. To complete the structure, fill the octets of the cl and br atoms with the remaining 24 electrons. Lone pairs of electrons themselves are usually represented by dots around the atoms. Web to draw the lewis structure for sicl2br2, first identify the central atom (si). Choose a suitable central atom for the compound. Find the total valence electrons in sicl2br2 molecule. Here, the given molecule is sicl2br2. Each line represents a single bond, and the dots represent lone pairs of electrons. Web draw the lewis structure for sicl, br2. Web draw the lewis structure for sicl2br2. Include all lone pairs of electrons. Silicon (si) has 4 valence electrons, chlorine (cl) has 7 valence electrons, and bromine (br) has 7 valence electrons. Include all lone pairs of electrons. Draw the molecule by placing atoms on the grid and connecting them with bonds. Web in this article, “sicl2br2 lewis structure”, lewis structure drawing, hybridization, shape, formal charge calculation with some detailed explanations are discussed. Draw the molecule by placing atoms on the grid and connecting them with bonds. Web overall, the lewis structure for sicl2br2 can be drawn as: The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. To add the element si, either double click on any atom and type the. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Find the total valence electrons in sicl2br2 molecule. Firstly, silicon (si) is the least electronegative and is chosen as the central atom and the others are arranged around it. Draw the molecule by placing atoms on the grid and connecting them with bonds. Choose a. Web in this article, “sicl2br2 lewis structure”, lewis structure drawing, hybridization, shape, formal charge calculation with some detailed explanations are discussed briefly. In order to find the total valence electrons in a sicl2br2 molecule, first of all you should know the valence electrons present in silicon atom, chlorine atom as well as bromine atom. Valence electronic structures can be visualized. Web the lewis structure for the molecule sicl2br2 is drawn by following a set of guidelines. Your solution’s ready to go! This is done by adding the valence shell electrons of all the constituent atoms. Calculate the total number of valence electrons. Web overall, the lewis structure for sicl2br2 can be drawn as: To add the element si, either double click on any atom and type the element symbol, or access a periodic table of elements from the more more button button. It also provides the molecular geometry and bond angle for sicl2br2 as well. Here’s the best way to solve it. Lewis structures of molecular compounds. In order to find the total. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Find more chemistry widgets in wolfram|alpha. Therefore, the total number of valence electrons in sicl2br2 is: Web how do you draw a lewis structure for sicl_2br_2? Valence electronic structures can be visualized by drawing lewis symbols (for atoms and monatomic ions) and lewis structures (for. Include all lone pairs of electrons. Web this video shows you how to draw the lewis dot structure for sicl2br2. Sicl 2 br 2 is a tetrahedral compound with sp 3 hybridization. In order to draw the lewis structure of sicl2br2, first of all you have to find the total number of valence electrons present in the sicl2br2 molecule. Web. Draw the molecule by placing atoms on the grid and connecting them with bonds. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Here’s the best way to solve it. To add the element si, either double click on any atom and type the element symbol, or access a periodic table of elements from. Your solution’s ready to go! Silicon (si) has 4 valence electrons, chlorine (cl) has 7 valence electrons, and bromine (br) has 7 valence electrons. This is done by adding the valence shell electrons of all the constituent atoms. Web overall, the lewis structure for sicl2br2 can be drawn as: Draw the molecule by placing atoms on the grid and connecting them with bonds. Choose a suitable central atom for the compound. In order to draw the lewis structure of sicl2br2, first of all you have to find the total number of valence electrons present in the sicl2br2 molecule. Part a draw the lewis structure for sicl2br2. To complete the structure, fill the octets of the cl and br atoms with the remaining 24 electrons. To add the element si, either double click on any atom and type the element symbol, or access a periodic table of elements from the more draw the molecule by placing atoms on the grid and connecting them with bonds. Sicl 2 br 2 is a tetrahedral compound with sp 3 hybridization. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Web draw the lewis structure for sicl, br2. Determine the total number of valence electrons in the molecule. Include all lone pairs of electrons. Find more chemistry widgets in wolfram|alpha.lewis structure for sicl2br2
Draw the Lewis Structure for Sicl2br2.
SiCl2Br2 Lewis Structure How to Draw the Lewis Structure for SiCl2Br2
Draw the Lewis structure for SiCl2Br2. HomeworkLib
lewis structure for sicl2br2
SiCl2Br2 Lewis Structure How to Draw the Lewis Structure for SiCl2Br2
Sicl2br2 Lewis Structure How To Draw The Lewis Struct vrogue.co
Draw The Lewis Structure For Sicl2br2
lewis structure for sicl2br2
Sicl2br2 Lewis Structure How To Draw The Lewis Structure
Valence Electronic Structures Can Be Visualized By Drawing Lewis Symbols (For Atoms And Monatomic Ions) And Lewis Structures (For Molecules And Polyatomic Ions).
View Available Hint(S) 0 Do Q?
Here’s The Best Way To Solve It.
Lone Pairs, Unpaired Electrons, And.
Related Post: